Data fraud of a nucleic acid testing institution in Beijing! Six people were subjected to criminal compulsory measures.
"Ping An Beijing" reported on the 21st that in response to the media reports and the public’s concern about the "Park Stone Medical Laboratory", the Health and Health Department has revoked the Laboratory’s Practice License for Medical Institutions, and the market supervision department has filed a case for investigation. According to the clues of the case transferred by the Health and Health Department, the public security organ filed a case for investigation on suspicion of obstructing the prevention and treatment of infectious diseases, and took criminal compulsory measures against six people including Zhou Moumou (male, 38 years old) and Wu Moumou (male, 37 years old), the actual controller of the laboratory, and the case is under further work. For such illegal and criminal acts, the public security organs will, together with the health and market supervision departments, find out and investigate them together, and crack down severely, and will never be soft.

Previously, the website of Fangshan District Government of Beijing released a message that Beijing Pushi Medical Laboratory Co., Ltd. was revoked the Practice License of Medical Institution by Fangshan District Health and Wellness Committee because the original test data was obviously less than the number of samples tested.
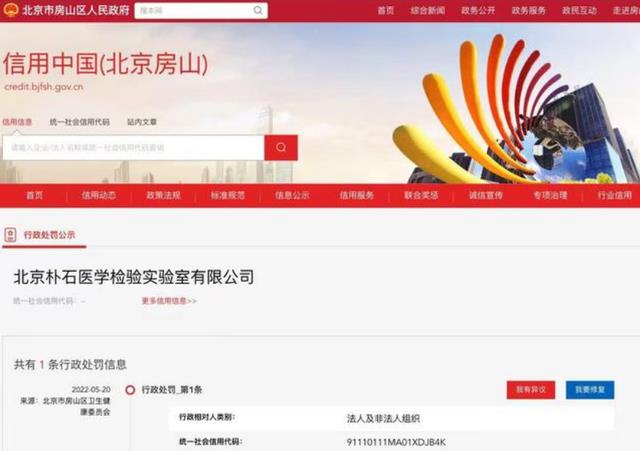
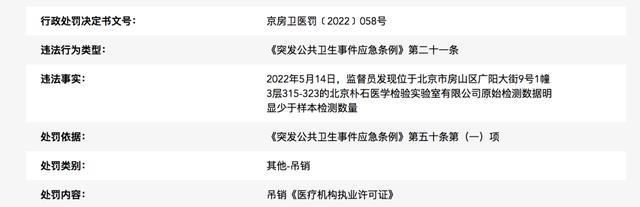
The public notice of administrative punishment shows that the illegal fact is May 14, 2022. The supervisor found that the original test data of Beijing Pushi Medical Laboratory Co., Ltd. located at Room 315-323, Floor 3, Building 1, No.9 Guangyang Street, Fangshan District, Beijing was obviously less than the number of samples tested.
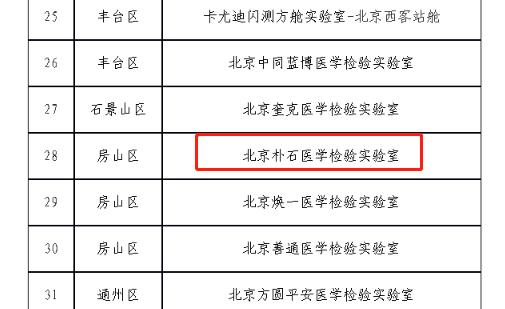
On May 9, Li Ang, deputy director and spokesperson of Beijing Municipal Health and Wellness Committee, informed at the press conference that during the recent flight supervision and inspection, some nucleic acid detection institutions found problems such as untimely inspection, inaccurate report and lax laboratory management, which seriously affected the quality of nucleic acid detection and the effect of epidemic prevention and control. In the list of qualified institutions audited by Covid-19 Nucleic Acid Detection Medical Laboratory in April 2022, Park Shi Medical is listed as one of the three nucleic acid institutions in Fangshan District.
In view of the existing problems, Beijing insists on problem-oriented, promptly investigates, corrects and rectifies them, and makes reforms and serious investigations, and mainly adopts four measures.
First, according to the law and regulations, the involved nucleic acid testing institutions will be dealt with seriously. At the first time, we talked with the legal representative of the organization, demanding that in-depth inspection and analysis be carried out, and the causes of the problems should be carefully found to prevent systematic problems in quality control management; This institution is required to suspend its practice, stop undertaking the nucleic acid testing business in Covid-19, and handle it according to the law and regulations according to the further investigation. The second is to draw inferences and carry out supervision and inspection of nucleic acid testing institutions in the city. Recently, in-depth and comprehensive supervision and inspection have been carried out on nucleic acid testing institutions in the city, and a mechanism of "case must be investigated" for positive case samples has been established to find hidden dangers, plug loopholes, adhere to problem orientation, comprehensively sort out problems, and prevent similar incidents from happening. The third is to strengthen cooperation and form a joint force of safety and quality supervision. Strengthen industry management and communication and coordination, establish a joint supervision mechanism for nucleic acid inspection quality in conjunction with drug administration, public security and other departments, carry out the "bright sword" special governance action, further strengthen the quality supervision of Covid-19 nucleic acid testing laboratory, improve the workflow, and strengthen the process audit; Establish a regular supervision mechanism, conduct regular spot checks on personnel samples in key industries, conduct regular spot checks and cross-checks on samples submitted by key industries such as express delivery services, circulation management services and hotel services, and conduct regular special inspections and quality and safety spot checks on testing institutions. The fourth is to trace the whole process and establish a reward and punishment mechanism for quality evaluation. The whole process of suspicious cases and positive samples detection should be traced back, and the institutions with standardized nucleic acid detection and strict quality control should be notified and praised, and the task of nucleic acid detection should be tilted.Institutions whose nucleic acid detection quality does not meet the standard shall be investigated and dealt with in informed criticism according to the law and regulations.
Li Ang said that the quality of nucleic acid detection is related to the overall situation of epidemic prevention and control. We will always adhere to the people first and life first, strictly control the quality of nucleic acid detection, strictly observe the bottom line of epidemic prevention work, and make every effort to protect people’s health and life safety.