Research and development of new diabetes drugs in 2022! Convolution intensified, and pharmaceutical companies joined forces to attack the 30 billion market.
China is a "big country", with more than 140 million diabetics, among whom about 72.83 million diabetics have not been diagnosed. Because diabetes requires lifelong medication, there is a huge demand for diabetes drugs in the market.
According to the Rong Yun database, the hospital sales of diabetes drugs (chemical drugs/biological drugs) in China increased from 20 billion yuan in 2014 to 34.3 billion yuan in 2021, with a compound annual growth rate of 7.99%.
Hospital sales of diabetes drugs market
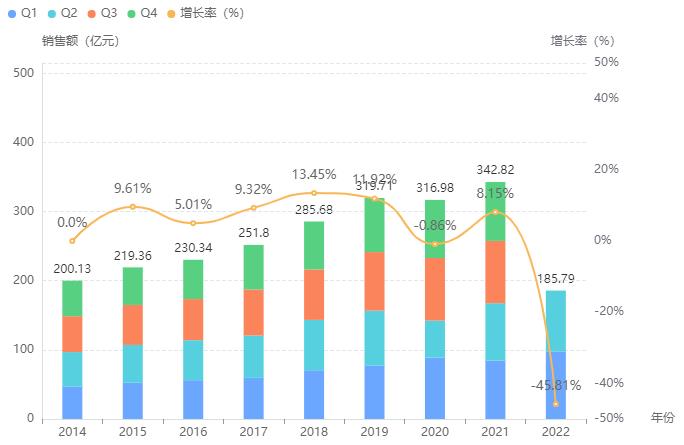
Screenshot Source: Drug Rong Yun National Hospital Sales Database
The market of diabetes drugs is 0.13 billion yuan, and the head effect is obvious.
Diabetes mellitus is a metabolic disease characterized by hyperglycemia in clinic, but the etiology and pathogenesis have not been fully understood at present. Its therapeutic drugs can be divided into two categories: non-insulin hypoglycemic agents and insulin hypoglycemic agents, and subdivided into ten categories: biguanides, insulin and its analogues, GLP-1 receptor agonists, sulfonylureas, glinides and GKA drugs. Jost Sullivan report shows that the current antidiabetic drug market in China is mainly occupied by traditional drugs, and half of it is occupied by insulin.
According to the statistics of Rong Yun Drug Database, among the TOP10 varieties in the hospital sales of diabetes drugs in 2021, except Dapagliflozin tablets, sitagliptin phosphate tablets and liraglutide injection, the other seven varieties are all insulin and its analogues.
Insulin aspart 30 injection and insulin glargine injection topped the list with sales exceeding 3 billion yuan.
TOP10 varieties of in-hospital sales of diabetes drugs market in 2021
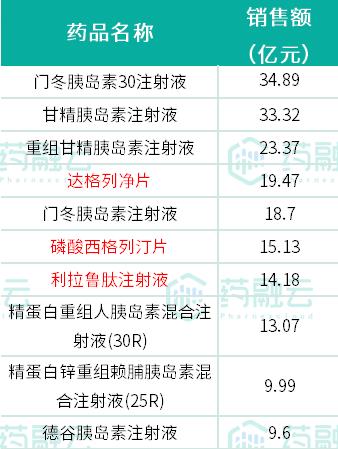
Source: Rong Yun National Hospital Sales Database.
The new diabetes drugs listed in 2022 are remarkable.
Compared with 2021, Hengrui Pharma’s Henggliclazide, Microchip’s Sigliclazide, Tanabe Mitsubishi Pharmaceutical’s Tegliclazide and many other new drugs were approved in China one after another. This year, fewer new diabetes drugs were approved, but all of them were remarkable and very eye-catching.
Hualing Medicine:Dogleetin tablets
On October 8, dorzagliatin, the first in class hypoglycemic agent of Hualing Medicine, was officially approved by NMPA for listing, with the trade name of Huatangning. Two indications of Huatangning were approved at one time, namely, using metformin alone to treat patients with type 2 diabetes who have not been treated with drugs, and using metformin alone in combination with metformin to treat adults with type 2 diabetes when blood sugar control is not good.
This is the world’s first approved GKA,Glucokinase Activator (GKA), the first original new drug with a brand-new mechanism in the field of diabetes in the past decade, and the first new drug for type 2 diabetes in the world launched in China for the first time. As a GKA drug, Dogleetin can restore the blood glucose homeostasis of patients with type 2 diabetes by restoring the function of glucose kinase (GK), and achieve the purpose of treating type 2 diabetes.
Lilly:Tirzepatide; tirzepatide
In May, 2022, FDA approved the listing of tirzepatide (trade name: Mounjaro), a dual agonist of glucose-dependent insulinotropic peptide (GIP) and glucagon-like peptide -1(GLP-1) receptor from Lilly. This drug is the first GLP-1R dual-target hypoglycemic drug for treating type 2 diabetes, and it is only injected once a week to assist diet and exercise, so as to improve the blood sugar control of adults with type 2 diabetes.
Research and development status of tirzepatide
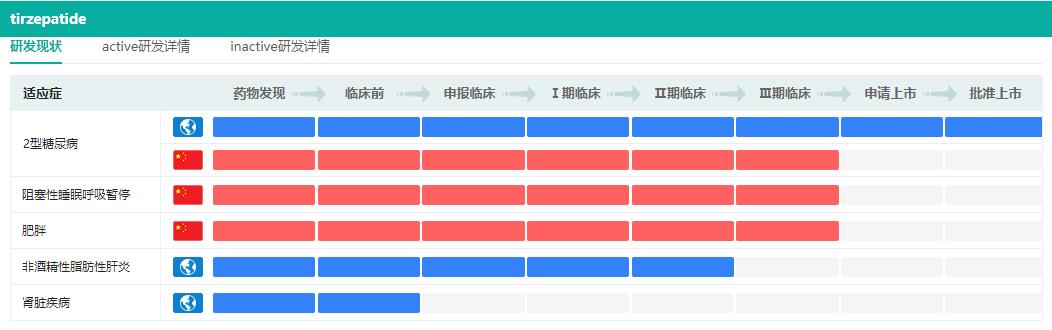
Screenshot Source: Drug Rong Yun Global Drug R&D Database
In China, Lilly submitted the IND application for Telpotide Injection for the first time in April 2019, and started clinical trials in August of the same year. So far, a number of phase III clinical trials for type 2 diabetes, heart failure and obesity have been carried out; In September this year, Lilly officially submitted the marketing application of the new diabetes drug to CDE.
Tirzepatide clinical trial information (search "Medicine Rong Yun applet" for more relevant data query)
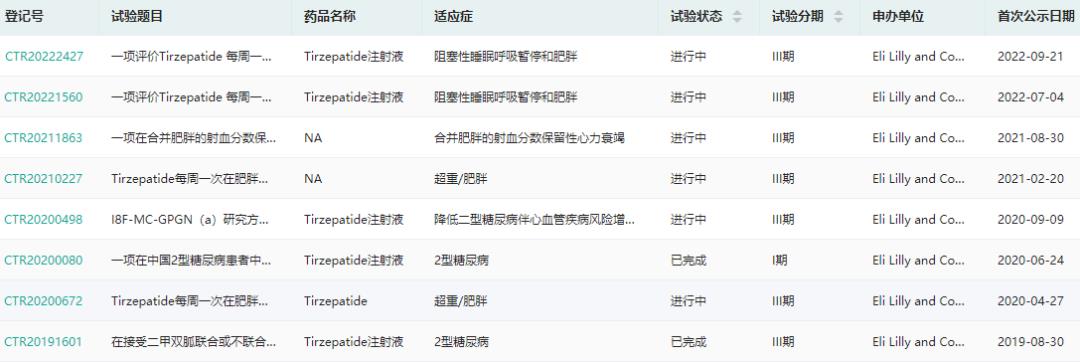
Screenshot Source: Drug Rong Yun China Clinical Trial Database
The research and development market of diabetes drugs has intensified, pending the inventory of new drugs by listed stars.
Under the policy of normalization of quantity procurement and consistency evaluation, the competition in the domestic diabetes generic drug market is gradually becoming fierce, and at the same time, it also forces domestic enterprises to move from generic drugs to Fast-follow and First-in-class.
The research and development of pharmaceutical companies has been accelerated, and many pharmaceutical companies have gathered together hot targets to compete for involution. At present, a number of new diabetes drugs have entered the phase III clinical stage, and some products have submitted listing applications in China.
Newly declared new diabetes drugs in China
Up to now, in addition to the telpotide mentioned above, there are several new diabetes drugs that have been submitted for marketing in the domestic diabetes drug market, which are worthy of attention.
Sihuan medicine:Jiaglinide tablets
On February 28th, the application for the listing of Jiagelijing tablets, a new drug of Sihuan Medicine, was accepted by CDE. This is a glucose sodium cotransporter 2(SGLT-2) inhibitor independently developed by Sihuan Medicine for the treatment of diabetes, which has been patented in China, the United States, Europe, Japan and South Korea for the treatment of type 2 diabetes. If approved for marketing, it will become the second SGLT-2 inhibitor drug independently developed by China after Hengrui Proline Hengglinide Tablets.
Novo Nordisk:Smegliptide tablets
On May 27th, Novo Nordisk’s application for the listing of Smegliptide Tablets was accepted by CDE. Smegliptide is a GLP-1 receptor agonist. Its injection has been approved for marketing in China, and its tablet has been approved for blood sugar control in adults with type 2 diabetes overseas, making it the first oral GLP-1 receptor agonist (GLP-1RA) in the world. According to the Rong Yun database, two phase III clinical trials of Smegliptide tablets compared with placebo or sitagliptin in the treatment of patients with type 2 diabetes have been completed. It is speculated that the declared indication is to treat type 2 diabetes.
Clinical trials of smeagoutide tablets in China
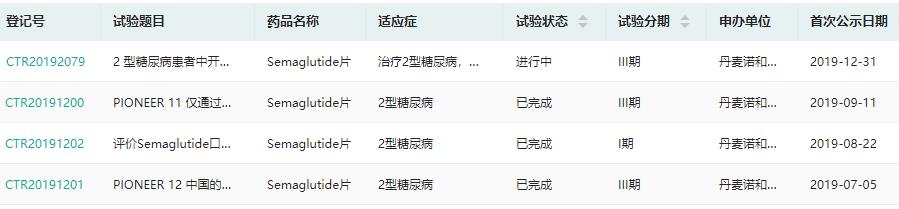
Screenshot Source: Drug Rong Yun China Clinical Trial Database
Hengrui Pharma:Repagliptin phosphate tablets
Repagliptin phosphate tablets in Hengrui Pharma, a DPP-4 inhibitor with similar structure to sitagliptin on the market, are also suitable for type 2 diabetes. It was submitted to the market in September 2020 and accepted. Proline Henggliclazide tablets reported at the same time were approved for marketing on the first day at the end of 2021, becoming the first SGLT-2 diabetes drug independently developed in China. Whether repaglinide can be approved before the end of 2022 is of concern.
Studying new drugs for diabetes.
Novo Nordisk:Icodec
In addition, no weekly insulin preparation products have been approved for listing in the world, and Novo Nordisk’s Icodec insulin is ahead of schedule. On October 3rd, Novo Nordisk announced the positive results of Icodec’s phase IIIa ONWARDS 5 study, and Novo Nordisk also said that it is expected to apply for regulatory approval of Icodec in the United States, the European Union and China in the first half of 2023.
Cinda Bio:Mazdutide(IBI362)
GLP-1 receptor agonists have grown rapidly in recent years, and have become the non-insulin drugs with the highest market share in the global diabetes market, among which dulaglutide, somaru peptide and liraglutide are outstanding. The multi-target agonist of GLP-1R has also become a hot competitive direction in the research and development of new drugs for diabetes.
Among them, the Mazdutide(IBI362) jointly promoted by Cinda Bio and Lilly is eye-catching. This is a GLP-1R/GCGR dual-target agonist, and its research on type 2 diabetes has reached the phase II clinical stage. On July 19th, Cinda Bio announced that a multi-center, randomized, placebo/dulaglutide-controlled phase II clinical study of IBI362 in type 2 diabetic subjects in China reached the main end point, with remarkable hypoglycemic and weight-loss effects, which can bring comprehensive benefits to patients. On October 4th, Cinda Bio announced the phase III clinical trial of IBI362 for overweight or obesity.
Dongyang Guangyao:Rong ge lie Jing
SGLT-2 target is one of the hot targets in the research and development of new drugs for diabetes in recent years. SGLT-2 inhibitors can block and reduce the reabsorption of glucose by the kidney, thus increasing glucose excretion through urination and lowering blood sugar level.
At present, among the SGLT-2 inhibitors under research, Rongglinide of Dongyangguang has made the fastest progress and has reached the clinical stage III. Tagglinide from Tianjin Institute of Medicine, Vanglinide from Fosun Pharma and Agglinide from Shanghai Alice have also entered the clinic.
A variety of clinical phase III DPP-4 inhibitors
There are many DPP-4 inhibitors in the domestic diabetes drug market, among which DBPR108 from Unacon, Fugliptin from Xinlitai, Shenggliptin from Shengshi Taike, HSK7653 from Haisike and Eugliptin from Yuandong Bio have made rapid progress, and all of them have reached the phase III clinical stage.
Zhengda Tianqing Nanjing Shunxin has laid out two models, TQ-F3083 and TQ05510, and increased the horsepower. The former is in the second clinical stage.
Both of them are DPP-4 inhibitors, and there are differences among different varieties. At present, DPP-4 inhibitors are mostly taken once a day, of which HSK7653 of Haisike Pharmaceutical is taken orally once every two weeks, and Eugliptin of Yuandong Biological and Borgliptin of Baidichang Pharmaceutical need to be taken once a week.
Finally, whether it is insulin or non-insulin diabetes drugs, we all look forward to the early listing to provide more choices for diabetic patients.